"Properties of Acids and Bases Lab"

 |
Biology 1 "Properties of Acids and Bases Lab" |
 |
Background Information:
Acids and bases are compounds that dissolve in water to give solutions with specific properties. Acids and bases are identified by their unique properties. Physically, both are able to conduct electricity and strong acids and strong bases are caustic to organic materials. Caution should be used when handling strong acids and bases because acid or base burns can occur. If you happen to spill any acid or base onto your skin during today's lab, make sure to rinse it under a cold water flow for a couple minutes.
Properties of Acids:
Acids cause foods and beverages to have a "sour" taste
Acids turn litmus paper red.
Phenolphthalein remains colorless when contacting acids.
Acids will react with certain metals. During these reactions, hydrogen gas (H2) will be released.
Acids will react with carbonates (like baking soda). During these reactions carbon dioxide (CO2) will be released.
Acids will have more hydronium (H+) ions in solution than hydroxide (OH-) ions.
Common compounds that are referred to as acids:
hydrochloric acid (HCl)
nitric acid (HNO3)
acetic acid (CH3COOH)
sulfuric acid (H2SO4)
vinegar (CH3COOH)
Properties of Bases:
Bases have a smooth, slippery feel to them when touched.
Bases have a bitter taste.
Bases turn litmus paper blue.
Phenolphthalein turns bright pink in the presence of bases.
Bases neutralize acids when mixed together.
Bases have more hydroxide (OH-) ions in solution than hydronium (H+) ions.
Common Compounds that are referred to as bases:
sodium hydroxide (NaOH)
potassium hydroxide (KOH)
ammonia (NH3)(aq)
baking soda (NaHCO3)
limewater or calcium hydroxide (Ca(OH)2(aq)
In this activity, you will be testing acids and bases to determine their affects on indicator dyes and litmus paper. You will also be testing the effects of acids on a variety of metals. Finally you will be conducting a neutralizing reaction between a weak acid and a weak base, observing what the results will be.
Caution: This lab will require the use of safety glasses. These must be worn when working with acids and bases. Acids and bases are caustic and can cause burns to the skin. Please be careful when working with these chemicals. Follow directions precisely and listen to the directions given by the instructor.

Materials Needed:
safety glasses
chem-plate
phenolphthalein indicator solution
litmus paper
Erlenmeyer flask
plastic cup
plastic spoon
notecard (3 x 5)
small balloon
plastic tray (all materials are in this tray)
electronic pan balance
paper towels
Acids & Bases Samples:
hydrochloric acid (HCl)
sodium hydroxide (NaOH)
acetic acid (CH3COOH)
baking soda (NaHCO3)
limewater solution (Ca(OH)2(aq)
30 ml sample of acetic acid (will be measured and given to you when you need it)
Metal Samples:
aluminum (Al)
copper (Cu)
magnesium (Mg)
zinc (Zn)
Procedure:
You will need to open the "Properties of Acids & Bases Lab Review" on Jupiter Ed and answer the questions as you go through the lab procedure.
Part One: Testing Acids with Litmus Paper and Phenolphthalein Solution
Place 4 drops of hydrochloric acid (HCl) in a chem-plate cavity. Test this with litmus paper by dipping one end of the litmus paper into the hydrochloric acid. Answer question #1 on your review. Throw away the litmus paper in the garbage when you are done using it.
Place 1 drop of phenolphthalein solution in the chem-plate cavity that has the hydrochloric acid in it. Answer question #2 on your review.
Place 4 drops of acetic acid into another empty chem-plate cavity. Test this with litmus paper by dipping one end of the litmus paper into the acetic acid. Answer question #3 on your review. Throw away the litmus paper in the garbage when you are done using it.
Place 1 drop of phenolphthalein solution in the chem-plate cavity that has the acetic acid in it. Answer question #4 on your review.
Rinse you chem-plate in the designated sink and dry it with paper towels.
Part Two: Testing Bases with Litmus Paper and Phenolphthalein Solution
Place 4 drops of sodium hydroxide (NaOH) into a clean cavity on your chem-plate. Test this with litmus paper by dipping one end of the litmus paper into the sodium hydroxide. Answer question #5 on your review. Throw away the litmus paper in the garbage when you are done using it.
Place 1 drop of phenolphthalein solution in the chem-plate cavity that has sodium hydroxide in it. Answer question #6 on your review.
Place 4 drops of limewater solution into another empty chem-plate cavity. Test this with litmus paper by dipping one end of the litmus paper into the limewater solution. Answer question #7 on your review. Throw away the litmus paper in the garbage when you are done using it.
Place 1 drop of phenolphthalein solution in the chem-plate cavity that has limewater solution in it. Answer question #8 on your review.
Rinse you chem-plate in the designated sink and dry it with paper towels.
Part Three: How Does Hydrochloric Acid React With Different Metals
Place a small amount (or chunk) of the following metals in separate cavities on your chem-plate: Aluminum, Copper, Zinc, and Magnesium. Make sure you remember which metal is in which cavity so you don't confuse your results.
Add 5 drops of hydrochloric acid to the cavity that contains the magnesium. Observe what occurs for a minute or so, and then answer question #9 on your review.
Add 5 drops of hydrochloric acid to the cavity that contains the zinc. Observe what occurs for a minute or so, and then answer question #10 on your review.
Add 5 drops of hydrochloric acid to the cavity that contains the aluminum. Observe what occurs for a minute or so, and then answer question #11 on your review.
Add 5 drops of hydrochloric acid to the cavity that contains the copper. Observe what occurs for a minute or so, and then answer question #12 on your review.
Dispose of the entire chem-plate into the garbage can as directed by Mr. Breitkreutz. Cleaning these trays would be pointless after the chemical reactions have damaged some of the cavities. DO NOT RINSE THESE CHEM-PLATES BEFORE DISPOSING THEM INTO THE GARBAGE.
Answer questions #13 thru #20 on your review.
Part Four: Using a Base to Neutralize an Acid
Obtain a beaker containing 30 ml of acetic acid from the lab table in the front of the classroom. Pour this 30 ml of acetic acid into the Erlenmeyer flask. Return this beaker to the lab table up front.
Using the spoon, plastic cup, and electronic pan balance, measure out a total of 25 g of baking soda.
Transfer the baking soda into the balloon using a 3 x 5 notecard to funnel the baking soda into the balloon. This is probably a 2-person job.
Place the balloon over the top of the Erlenmeyer flask as seen below:
Carefully tip the balloon up so that the baking soda falls into the acetic acid. Observe what happens for a minute or so, and then answer question #21 on your review.
The chemical reaction that occurs when you combine acetic acid and baking soda is shown below:
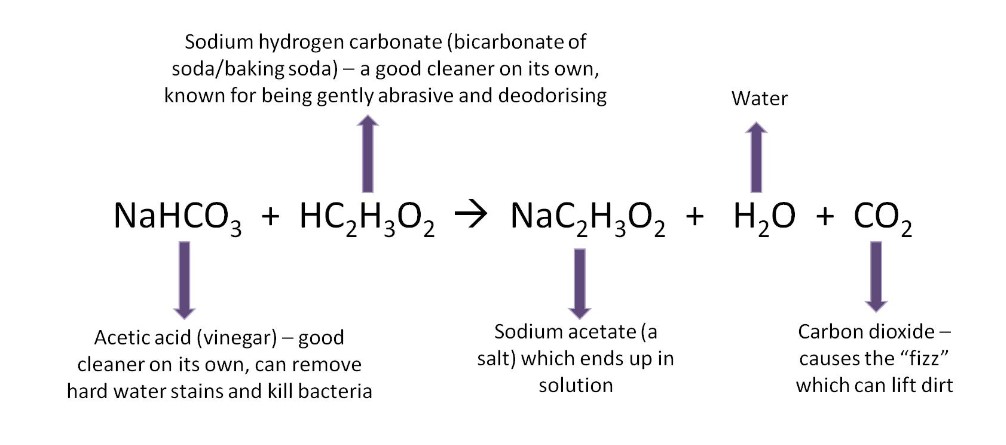
At the end of reaction this is what you will end up with:
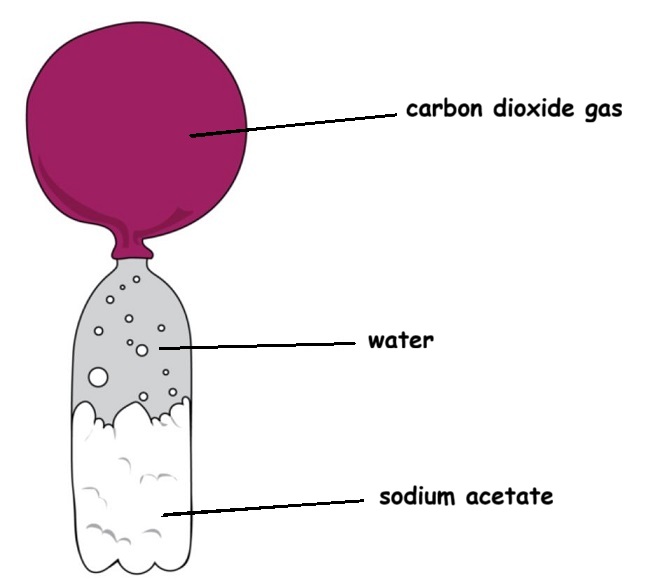
Rinse out the erlenmeyer flask and dry it as best you can. Getting all of the gunk out of it is the main objective.
Clean up your designated areas and the counter tops by the sinks. Make sure to clean the tops of the electronic pan balances (the metal top does come off and you can clean it more thoroughly). Replace all materials back in the tray and make sure that everything is in order just as you found it. The only thing you will throw away is the used chem-plate. The spoon, plastic cup, and notecard can be reused because they were only used for dry materials.
You can now answer the rest of the questions (Questions #27 - #33) on your lab review. Check the marker board in the Biology classroom for the exact due date for this assignment.