(n-butyl)
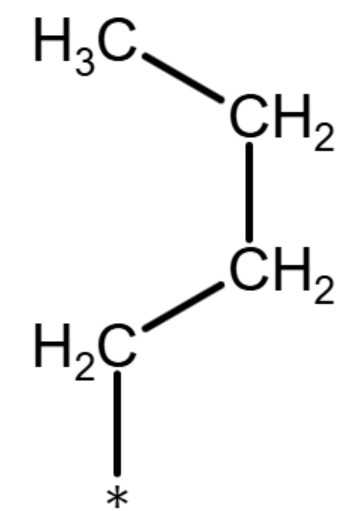
(sec-butyl) or (s-butyl)
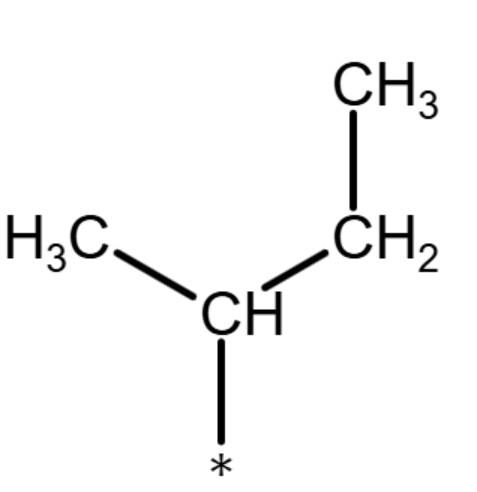
(tert butyl) or (t-butyl)
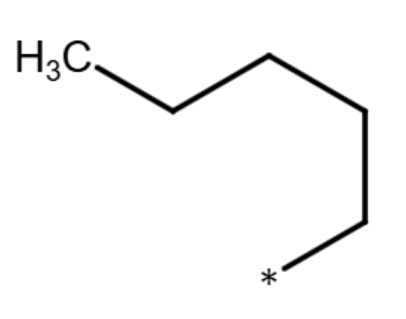
(n-pentyl group)
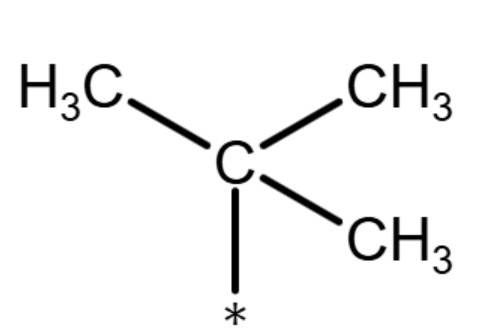
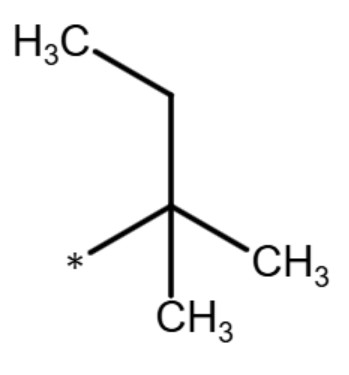
(t-pentyl group)
Organic Chemistry
Understanding Prefixes and Functional Groups
Common Prefixes Used to Identify Carbon Parent Chains
meth = 1 carbon atom
eth = 2 carbon atoms
prop = 3 carbon atoms
but = 4 carbon atoms
pent = 5 carbon atoms
hex = 6 carbon atoms
hept = 7 carbon atoms
oct = 8 carbon atoms
non = 9 carbon atoms
dec = 10 carbon atoms
*for larger numbers see Table 11.2 on page 321 of your Organic Chemistry Textbook
Common Alkyl Groups Used as Substituent Groups with Organic Molecules
| Alkyl Group Name | What Does the Akyl Group Look Like? |
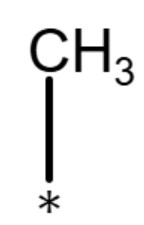
| methyl group |
 |
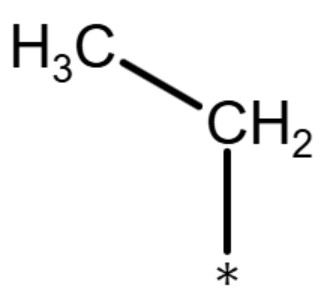
| ethyl group |
 |
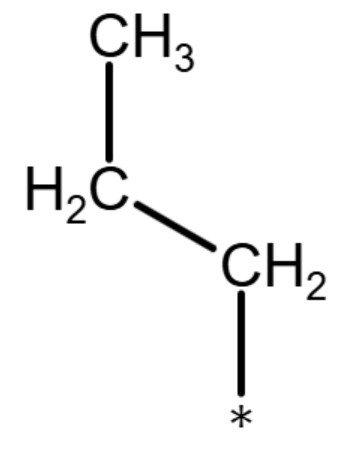
| propyl group |
 |
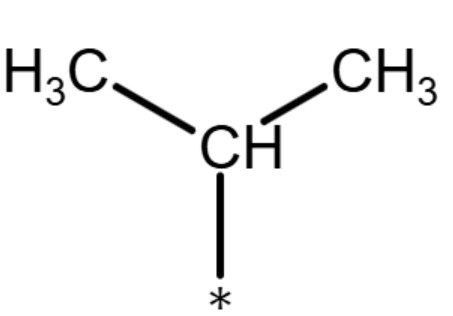
| isopropyl group |
 |
| normal
butyl group (n-butyl) |
 |
|
secondary butyl group (sec-butyl) or (s-butyl) |
 |
| tertiary
butyl group (tert butyl) or (t-butyl) |
 |
| normal
pentyl group (n-pentyl group) |
 |
| tertiary
pentyl group (t-pentyl group) |
 |
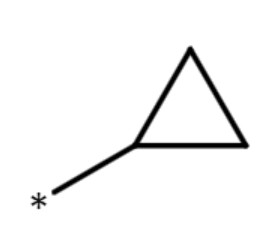
| cyclopropyl group |
 |
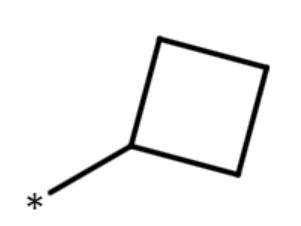
| cyclobutyl group |
 |
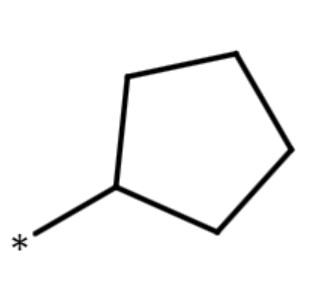
| cyclopentyl group |
 |
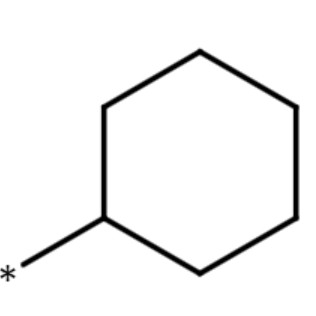
| cyclohexyl group |
 |
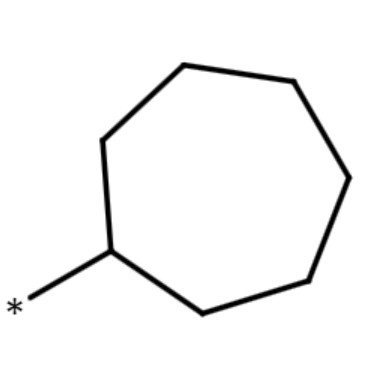
| cycloheptyl group |
 |
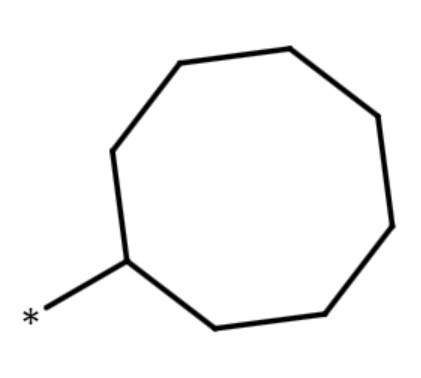
| cyclooctyl group |
 |
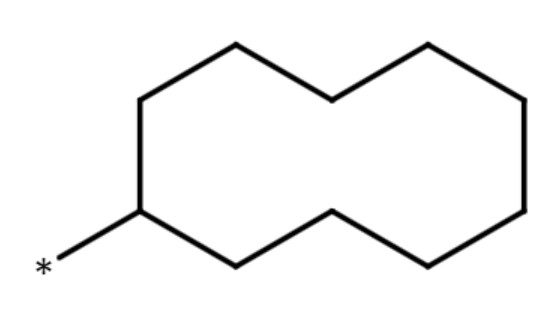
| cyclodecanyl group |
 |
*You can keep going with hexyl groups, heptyl groups, etc. and the naming process will continue.
Explanation for the terms "Normal", "Secondary", "Tertiary" and "Iso"
Normal (n-)
The term "normal" refers to an alkyl group that is attached to the end carbon of the alkyl group and forms a continuous chain. Chemists often just leave off the "normal" or "n-" distinction when naming organic compounds with the understanding if no name is given for the substituent group that the "normal" group is used. See the diagram below for an example:
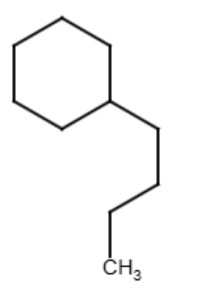
butylcyclohexane
One does not need to name it n-butylcyclohexane because it's understood that with no distinct name given for the alkyl group that it will be the normal alkyl group.
Secondary (sec-) or (s-)
The term "secondary" refers to a functional group where the carbon that attaches to the parent chain is also connected to two other carbons in the functional group. The term is used for functional groups that have more than 3 carbons in the functional group such as butyl, pentyl, hexyl, etc. See the diagrams below for examples:
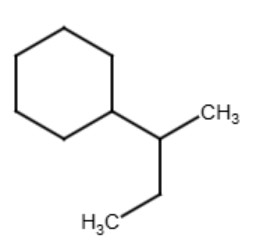 |
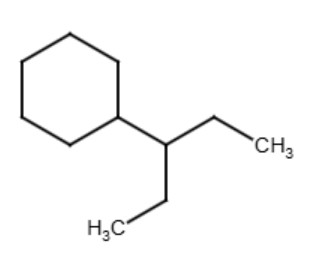 |
| sec-butylcyclohexane | sec-pentylcyclohexane |
Tertiary (tert-) or (t-)
The term "tertiary" refers to a functional group where the carbon atom that attaches to the parent chain is also connected to three other carbon atoms that are part of the functional group. The term is used for functional groups that have more than three carbons in the functional groups similar to how the term "secondary" is used. See the diagrams below for examples:
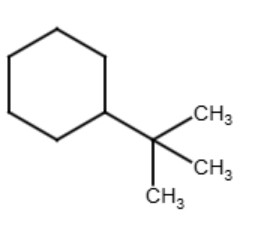 |
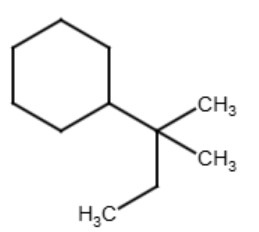 |
| tert-butylcyclohexane | tert-pentylcyclohexane |
Alphabetizing and Hyphen Rules
The terms "secondary" (sec-) and "tertiary" (tert-) are treated as follows:
1. When using sec- or tert- in a name, the terms are considered part of the name of the functional group and will be alphabetized by using the "s" or "t". The term "meth" starts with the letter "m" so it would be listed before the sec- or tert- terms in the IUPAC naming process.
2. Hyphens ensure that the prefix is recognized as a separate part of the name, preventing misinterpretation, particularly when the following word starts with a vowel. So use hyphens after the word "sec-" or "tert-".
See the example below for clarification of the above rules:
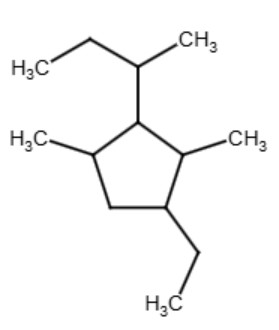 |
| 1-ethyl-2,4-dimethyl-3-sec-butylcyclopentane |
The ethyl group is listed first, then the methyl group, followed by the sec-butyl group
*Question: Why does the numbering start with the ethyl group and not the methyl group?
*Answer: The ethyl group has more carbon atoms than the methyl group so it takes priority.
*Question: Why not start start the numbers on the sec-butyl group, it has the most carbons?
*Answer: If you start on the sec-butyl group and go clockwise you get the numbers 1,2,3,5 (added together you get 11). If you start on the sec-butyl group and go counter-clockwise you get the numbers 1,2,4,5 (added together you get 12). But if you start with the ethyl group you get the numbers 1,2,3,4 (added together you get 10) which is the lowest number possible for your substituent groups.