
Properties of Water Lab

Introduction
With 70% of our earth being ocean water and 65% of our bodies being made up of water, it is hard to not be aware of how important it is in our lives. This simple fact is why scientists are constantly looking for water on other planets - the presence of water could indicate the presence of life. There are three different forms of water, or H2O: solid (ice), liquid (water), and gas (steam). Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:
Polarity
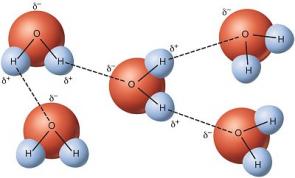
Water has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms. Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons. Oxygen also has two unshared pairs of electrons. Thus there are 4 pairs of electrons surrounding the oxygen atom, two pairs involved in covalent bonds with hydrogen, and two unshared pairs on the opposite side of the oxygen atom. Oxygen is an "electronegative" or electron "loving" atom compared with hydrogen.
Water is a "polar" molecule, meaning that there is an uneven distribution of electron density. Water has a partial negative charge (δ-) near the oxygen atom due to the unshared pairs of electrons, and partial positive charges (δ+) near the hydrogen atoms.
An electrostatic attraction between the partial positive charge near the hydrogen atoms and the partial negative charge near the oxygen results in the formation of a hydrogen bond. The ability of ions and other molecules to dissolve in water is due to polarity. Many other unique properties of water are due to the hydrogen bonds. For example, ice floats because hydrogen bonds hold water molecules further apart in a solid than in a liquid, where there is one less hydrogen bond per molecule. The unique physical properties, including a high heat of vaporization, strong surface tension, high specific heat, and nearly universal solvent properties of water are also due to hydrogen bonding. The hydrophobic effect, or the exclusion of compounds containing carbon and hydrogen (non-polar compounds) is another unique property of water caused by the hydrogen bonds. The hydrophobic effect is particularly important in the formation of cell membranes. The best description is to say that water "squeezes" non-polar molecules together.
Boiling and Freezing Points
The ability of water molecules to form hydrogen bonds, as shown below, causes many of water's unique characteristics. Despite having a small molecular weight, it has an incredibly big boiling point (100°C). This is because water requires more energy to break its hydrogen bonds before it can then begin to boil. The same concept is applied to the freezing point as well. The boiling and freezing points of water enable the molecules to be very slow to boil or freeze, this is important to the ecosystems living in water. If water was very easy to freeze or boil, drastic changes in the environment would affect bodies of water such as oceans or lakes, and causee all the organisms living in water to die. This is also why sweat is able to cool our bodies. Let's look at how water is different than most compounds in regards to its boiling and freezing points:
| Compound | Boiling Point | Freezing Point |
| Hydrogen Telluride | -4°C | -49°C |
| Hydrogen Selenide | -42°C | -64°C |
| Hydrogen Sulfide | -62°C | -84°C |
| Water | 100°C | 0°C |
Converting temperatures from Celsius to Fahrenheit is accomplished by following the formula below:
(ºC. x 1.8) + 32 = ºF.
Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature
of one gram of a substance by one degree Celsius. Every substance has its
own specific heat capacity, with the specific heat capacity of water being 1.0
cal/(g°C).
The specific heat capacity of water is much higher than that of other common
substances. For the sake of comparison, the specific heat capacity of oil
is about 0.5 cal/(g°C) and the specific heat capacity of aluminum is about 0.2
cal/(g°C). This means that it takes a lot more heat to raise the
temperature of water compared to the amount of heat it would take to raise the
temperature of oil or aluminum.
The high specific heat of water helps the earth's temperature remain moderate
since water traps heat during the day and releases it slowly at night. As
a result, the temperature on earth's surface does not vary very widely, ranging
from extremes of 134°F to -129°F. For comparison, the moon has no liquid
water and its temperatures can range from 240°F to -290°F. (The lack of
atmosphere on the moon, along with other factors, also contributes to the wide
range of temperature.)
Density
Density is the ratio of mass to volume and dense objects feel heavier and tend to sink, while less dense objects feel lighter and tend to float. The density of most objects changes slightly as the temperature changes. In general, warmer temperatures tend to make substances less dense because the greater random kinetic energy makes the molecules spread out. The amount that objects expand when heated is known as the coefficient of expansion.
The formula for calculating density is shown below. The answer is expressed in grams per centimeters cubed or (g/cm3)
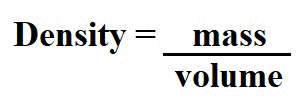
The density of water, once again, is a special case. Water is most dense
at 39°F, and as it cools or warms from this temperature, the water expands
slightly. This means that ice is slightly less dense than cold water,
which is why ice floats on the surface of bodies of water. The floating
ice slows the freezing process by insulating the water underneath, which
contributes to the moderate temperatures on earth. In addition, the layer
of ice prevents many lakes from freezing solid, allowing fish and other
organisms to survive under the ice.
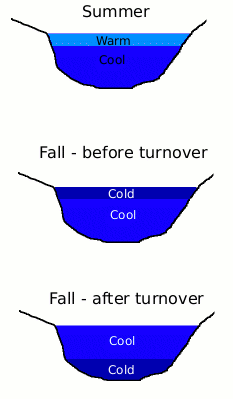
The changing density of water at different temperatures is also responsible for turnover. Turnover occurs when the water on the surface of a lake cools in the fall. Eventually, this cold water will become more dense than the warmer water beneath, so the cold water will sink to the bottom and the warm water will rise to the surface. When lakes are used as the water source for water treatment plants, turnover can cause abrupt changes in the quality of the raw water.
Surface Tension, Heat of Vaporization and Vapor Pressure
Besides mercury, water has the highest surface tension for all liquids. Surface
tension is the property of the surface of a liquid that
allows it to resist an external force, due to the cohesive nature of its
molecules. Since these forces vary depending on the nature of the liquid (i.e.
water vs. gasoline) or solutes in the liquid (surfactants like detergent), each
solution exhibits differing surface tension properties. In a body of water,
hydrogen bonds between water molecules are constantly pulling the molecules in
many different directions. However, at the water's surface, the molecules
are only being pulled from side to side and down, with no hydrogen bonds pulling
them upwards. This results in a skin of water at the surface in which the
molecules are held together very tightly.
Surface tension is a measurement of the amount of force required to break this
skin on the surface of water. Other liquids have a surface tension as
well, but the surface tension in water is quite strong due to the hydrogen
bonds. The pictures below show some examples of the results of water's
strong surface tension. Whether you know it or not, you already have seen
surface tension at work. Whenever you fill a glass of water too far, you may
notice afterward that the level of the water in the glass is actually higher
than the height of the glass. You may have also noticed that the water that you
spilled has formed into pools that rise up off the counter. Both of these
phenomena are due to surface tension.

Water's high surface tension is due to the hydrogen bonding in water molecules.
Water also has an exceptionally high heat of vaporization. Vaporization occurs when a liquid changes to a gas, which makes it an endothermic reaction. Water's heat of vaporization is 41 kJ/mol. Because the molecules of a liquid are in constant motion and possess a wide range of kinetic energies, at any moment some fraction of them has enough energy to escape from the surface of the liquid to enter the gas or vapor phase. This process, called vaporization or evaporation, generates a vapor pressure above the liquid. The heat of vaporization is the heat required to induce this phase change (liquid to gas).
Vapor pressure is inversely related to intermolecular forces, so those with stronger forces have a lower vapor pressure. Water has very strong intermolecular forces, hence the low vapor pressure, but it's even lower compared to larger molecules with low vapor pressures.
Viscosity is the property of fluid having high resistance to flow. We normally think of liquids like honey or motor oil being viscous, but when compared to other substances with like structures, water is viscous. Liquids with stronger intermolecular interactions are usually more viscous than liquids with weak intermolecular interactions.
Cohesion is the intermolecular forces between like molecules; this is why water molecules are able to hold themselves together in a drop.

Water molecules are very cohesive because of the molecule's polarity. This is why you can fill a glass of water just barely above the rim without it spilling.
Capillary Action
Plants and trees couldn't thrive without capillary action. It helps bring water up into the roots. With the help of adhesion and cohesion, water can work its way all the way up to the branches and leaves. Even if you've never heard of capillary action, it is still important in your life. Capillary action is important for moving water (and all of the things that are dissolved in it) around. It is defined as the movement of water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and ahesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus, which turns upward. The surface tension acts to hold the surface intact. Capillary action occurs when adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (below) is limited by surface tension.
| The attraction of water molecules to the sides of a narrow vessel (adhesion) is stronger than the cohesion drawing water molecules together. The result is capillary action, in which the force of adhesion pulls the fluid upwards. |
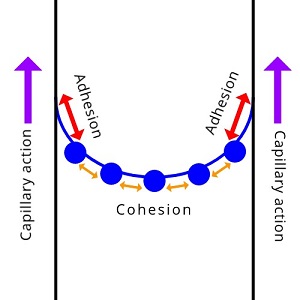 |

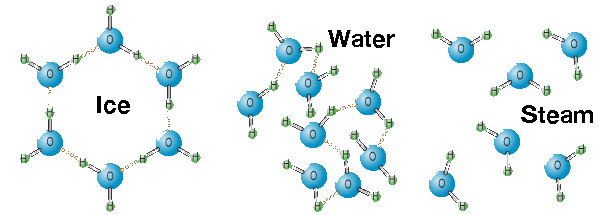
Solid State (Ice)
All substances, including water, become less dense when they are heated and more dense when they are cooled. So if water is cooled, it becomes more dense and forms ice. Water is one of the few substances whose solid state can float on its liquid state! Why? Water continues to become more dense until it reaches 4°C. After it reaches this temperature, it becomes less dense. When freezing, molecules within water begin to move around more slowly, making it easier for them to form hydrogen bonds and eventually arrange themselves into an open crystalline, hexagonal structure. Because of this open structure as the water molecules are being held further apart, the volume of water increases about 9%. So molecules are more tightly packed in water's liquid state than its solid state. That is why a can of soda can explode in the freezer.

Liquid State (Liquid Water)
It is very rare to find a compound that lacks carbon to be a liquid at standard temperatures and pressures. So it is unusual for water to be a liquid at room temperature! Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds resulting in the molecules being packed more closely together. Each water molecule links to four others creating a tetrahedral arrangement, however they are able to move freely and slide past each other, while ice forms a solid, larger hexagonal structure.
Water - Tetrahedral arrangement
Gas State (Steam)
As water boils, its hydrogen bonds are broken. Steam particles move very far apart and fast, so barely any hydrogen bonds have the time to form. So, less and less hydrogen are present as the particles reach the critical point above steam. The lack of hydrogen bonds explains why steam causes much worse burns than water. Steam contains all the energy used to break the hydrogen bonds in water, so when steam hits your face you first absorb the energy the steam has taken up from breaking the hydrogen bonds in its liquid state. Then, in an exothermic reaction, steam is converted into liquid water and heat is released. This heat adds to the heat of boiling water as the steam condenses on your skin.
Hydrogen bonds are strongest at subzero temperatures when they hold six oxygen atoms in a tight hexagonal molecule of ice. In its liquid form oxygen atoms are loosely linked in chains, whereas at temperature above 100°C, they are separated in steam.
The Universal Solvent
Water is called the universal solvent because more substances dissolve in water than in any other substance. This has to do with the polarity of each water molecule. The hydrogen side of each water molecule carries a slight positive electric charge, while the oxygen side carries a slight negative electric charge. This helps water dissociate (separate) ionic compounds into their positive and negative ions. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. Despite being called the universal solvent, water doesn't dissolve everything. Most of the hydroxides exhibit low solubility in water. Also, nonpolar molecules don't dissolve very well in water, including many organic compounds, such as fats and waxes. It is called the universal solvent because it dissolves the most substances, not because it dissolves every single compound.
Why Is This Important?
The properties of water make it suitable for organisms to survive in during differing weather conditions. Ice freezes as it expands, which explains why ice is able to float on liquid water. During the winter when lakes begin to freeze, the surface of the water freezes and then moves down toward deeper water; this explains why people can ice skate on or fall through a frozen lake. If ice was not able to float, the lake would freeze from the bottom up killing all ecosystems living in the lake. However ice floats, so the fish are able to survive under the surface of the ice during the winter. The surface of ice above a lake also shields lakes from the cold temperature outside and insulates the water beneath it, allowing the lake under the frozen ice to stay liquid and maintain a temperature adequate for the ecosystems living in the lake to survive.